My high school science class memories have absolutely nothing to do with science.
Salt water conductivity measurement.
Adjust sample temperature about 25 3.
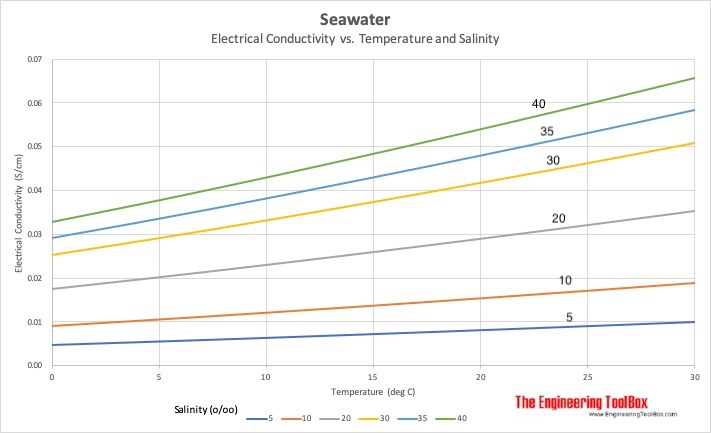
Conductivity is a key parameter for in situ measurements of several fundamental physical properties of salt water.
Immerse cell in sample.
Salt water conductivity specific conductance conductivity faqs.
Salinity is defined as the concentration of dissolved solids.
This ability is directly dependent on the concentration of conductive ions present in the water.
Read note conductivity of sample 5.
Let s go back to conductivity.
For saltwater the ability to conduct electrical current is mostly dependent on temperature and the amount of inorganic dissolved solids.
A measurement of 1 000 ec is also the same as 640 parts per million which is the unit that s used to determine how much salt is in your pool water.
Once you have this measurement 1 000 microsiemens per centimer is considered to be equivalent to 1 000 ec which is the electrical conductivity of water.
Calculate ec at 25 c.
Both my biology and chemistry teachers were known for incessant lectures and assigning endless research.
Conductivity is an index of how easy it is for electricity to flow.
Conductivity and salinity have a strong correlation 3.
Keep reading for instructions for a salt water conductivity experiment.
It can also be used to measure the salinity of water but a high quality ec meter may be significantly more expensive than a refractometer or hydrometer.
As conductivity is easier to measure it is used in algorithms estimating salinity and tds both of which affect water quality and aquatic life.
An electrical conductivity meter or ec meter is the only common device that can be used to measure the salinity of soil.
Salinity is important in particular as it affects dissolved oxygen solubility 3.
The conductivity of water is a measure of the capability of water to pass electrical flow.
Conductivity is a key parameter for in situ measurements of several fundamental physical properties of salt water.
Measure temperature of sample record to nearest 0 1 c 6.
Common table salt nacl is an electrolyte and when this is dissolved in water to form salt water it becomes sodium ions na and chloride ions cl each of which is a corpuscle that conducts electricity.
Sample level above vent holes 4.